eBMR for Pharma (Electronic Batch Manufacturing Record for Pharmaceutical) Manufacturing
Importance of eBMR for Pharma:
eBMR for Pharma is very critical for Pharma Manufacturing for various reasons. Even today, surprisingly, lot of big pharmaceutical manufacturing companies have been capturing the batch manufacturing details on paper. This paper based BMR involves lot of effort in printing all the process parametric data from different process steps of the batch manufacturing. Human beings have to walk across the manufacturing floor, collect these paper printouts, compile them and sometimes, they may have to repeat this process and manually got to verify the same by respective QA individuals. This is a cumbersome process
Where should one start the eBMR journey?
This process parametric data comes from wide variety of PLCs monitoring the processes. When I say, wide variety of PLCs, this means from different vendors, different models that got evolved a long period of 20-30 years. How do we bring all the data that is coming from these machines into an eBMR? What to do with existing investments that the manufacturers have made for the automation of the L1 and L2 layers of automation, where there is lot of HMI/SCADA. Lot of this machinery comes with the embedded PLCs and how do we ensure that these machines are 21 CFR Part 11 compliant? Now, the manufacturers’ have the dilemma, should they make each and every machine 21 CFR Part 11 compliant first or should they go for an eBMR solution? Is eBMR solution going to solve all the problems in one shot? There is also a concern from some of the manufacturers that eBMR solution is expensive and difficult to scale because of cost reasons and validations involved. What is the truth here? Upgrading of all the machinery on the floor for 21 CFR Part 11 compliance is not an option obviously, we all know that the new machinery comes with the 21 CFR Part 11 compliance but how to deal with the older machinery? Should the manufacturer invest into the HMI/SCADA automation? This is something, an option in the past but with the availability of proven new IIOT technologies, it does not make sense to invest into the HMI/SCADA now just for the eBMR purposes. HMI/SCADA has its own justification. With Atachi NGIMES-IIOT, NGIMES platform allows the pharma manufacturers to pull all the data seamlessly from wide variety of PLCs without having to do the additional investments like HMI/SCADA. Atachi NGIMES-IIOT not only pulls the data, displays the real-time process parametric data, even allows to change the process parameters via NGIMES-IIOT including switching on and off the machinery. Every feature can be implemented at manufacturers’ discretion with flexibility and compliance at every step.
What about the Data retention?
Unlike, other eBMR vendors, at Atachi we take the responsibility of data retention throughout the life of the product. During this whole life of the product (i.e., tablets, vaccines, etc.) the manufacturer does not have to worry about upgrading their eBMR platform and the associated reporting tools, data warehouses databases, etc. This is a big task and obviously expensive and tedious life software and hardware cycle management task. Atachi NGIMES provides the eBMR for any batch throughout the data retention period and beyond for continuous improvement and manufacturing insights.

What functionalities do you need as part of eBMR?
Today, lot of pharma manufacturers, especially Indian Pharma manufacturers capture lot of information in their BMR on paper. When they try to digitize this paper bmr into eBMR using the international eBMR platforms, this functionalities that they are capturing on paper is not available for one single cost or license. What does it mean? When you generate a BMR, the BMR template needs to be created at first and that needs to be reviewed and approved by QA department. This functionality is sold as a separate package by some MES vendors. As part of the preparation of the raw materials prior to production, warehouse performs weighing and dispensing. This functionality is offered as a standalone package by lot of MES vendors and small Indian software firms. When the pharma manufacturer starts deploying the functionality one by one, the integration of these functionalities becomes challenge. Some MES platforms have integration issues with the machinery on the production floors hence demanding the situation of retrofitting these machineries or upgrading the machineries with 3rd party OPC software or IIOT gateways. This is becoming a challenge for the Pharma manufacturers in digitizing their paper based BMR. This is the key differentiator with Atachi NGIMES platform, it is bundled with all the functionalities. We do not distinguish weighing and dispensing as a separate package, creating Master Batch Records or Recipe Management as a separate package. This is all included as part of the eBMR. Atachi NGIMES platform is a complete digitization platform with all the functionalities included, see the picture below:
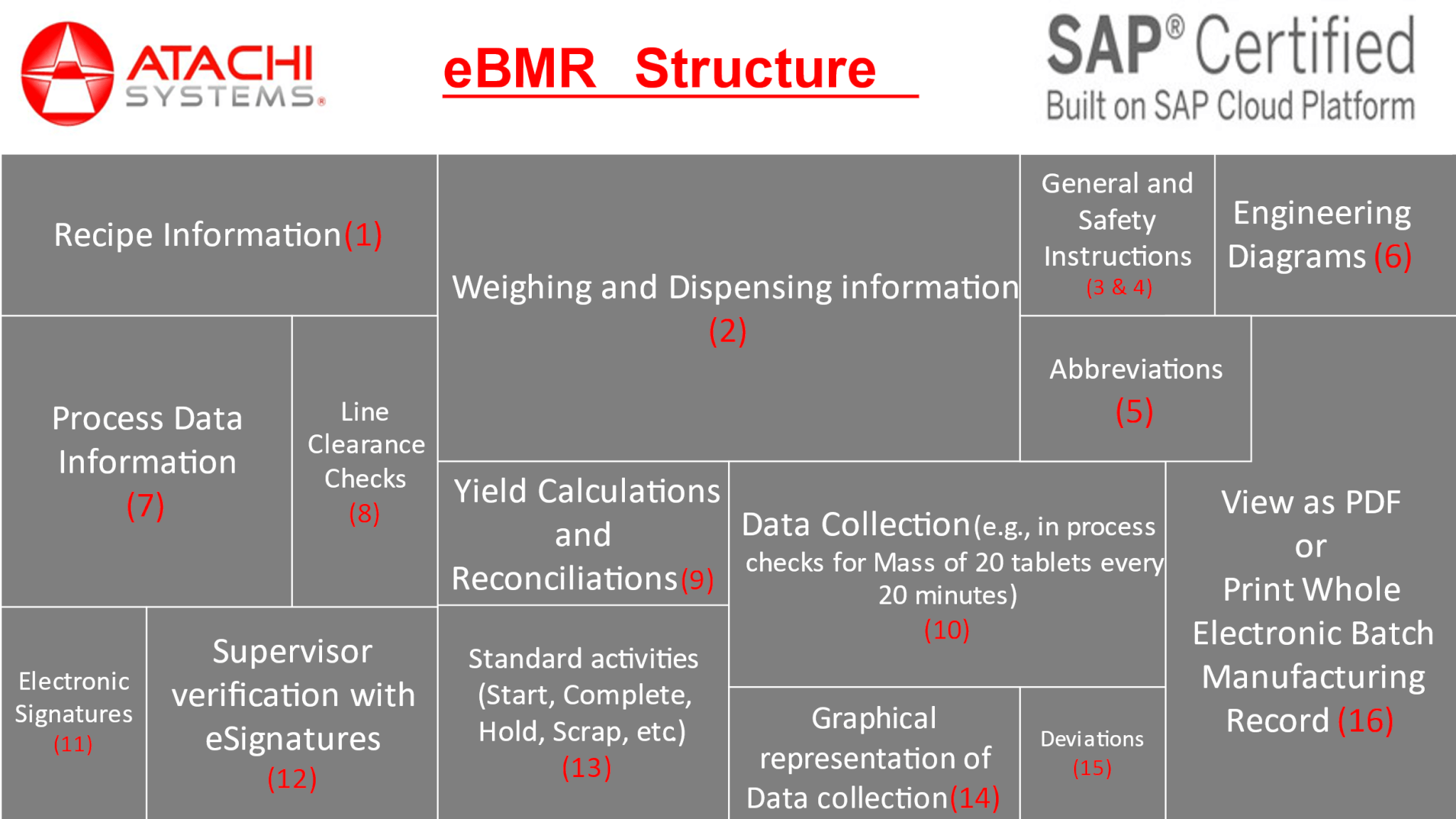
eBMR is not done yet!
What happens if a process parametric data is out of specification? We shall be able to generate the deviations and send out the alerts, take the actions in real-time. Now, do you need to go and buy a CAPA platform or QMS platform to take care of the deviations and change controls? What if the raw materials being received into the warehouse cannot accommodate the laptops? Do you need to pay for additional mobility to run the application on the mobile devices to scan the incoming raw materials for capturing the raw material information, sampling, and authorization. How do you verify the certificate of acceptance for raw materials in real-time? You should not be spending meaningless budgets for the integration of these MES platforms with 3rd party QC software and ERP systems. Atachi NGIMES seamlessly integrates with 3rd party software running either on-premise or cloud or hybrid model. Atachi NGIMES natively integrates with SAP ERP system, SAP S/4HANA as we built our NGIMES on SAP HANA technology.
How do you ensure that the associates are trained to work on the eBMR process steps?
Traditional MES platforms do not come with the training management system. How do you tightly ensure that the only certified operators are operating the required machinery and associated operations? With Atachi NGIMES – Training Management System (TMS), you can create your training documents including videos and let the associates take the tests and get them certified, certifications are automatically applied and enforced when the users logon to the system. Take a look at the complete Atachi NGIMES platform for Pharma Manufacturing, which is available for single license, user can go across all the functionalities as needed based on the configuration and customization done for the user’s manufacturing company and roles and privileges given to the associate.
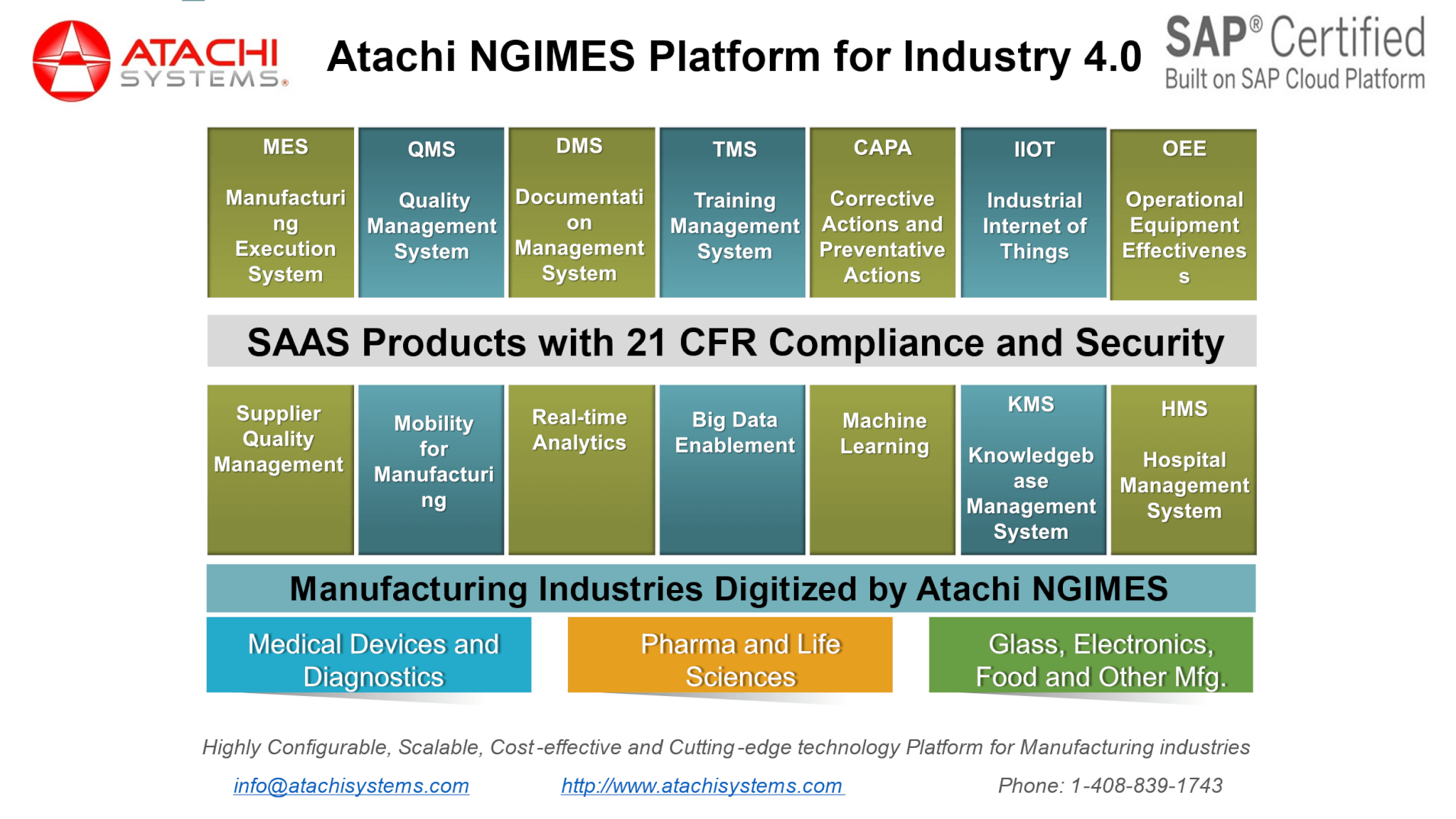
None of the above functionalities are less important, OEE for Pharma – elogbooks for Pharma:
A pharma manufacturer needs all the above functionalities for their eBMR. We all know that, but can we afford to buy all the above functionalities from different vendors, integrate them and maintain them throughout their independent software lifecycles? How do you ensure that the equipment cleanliness is performed as per the FDA guidelines? We still use lot of paper logbooks to capture the details and ensure that the cleanings are performed. This is significant and tedious task, which has been draining the lot of human productivity across pharma manufacturing and yet susceptible to human errors and data integrity issues. At, Atachi, eLogBooks for Pharma is part of the Atachi NGIMES platform. Here, elogbooks is not simply replacing bunch of user interface screens replacing paper, instead we generate the insights and help gain the productivity by increasing the production performance, machine availability and quality. One of our customers uncovered a hidden factory of 10 percent by leveraging our Atachi NGIMES- OEE for Pharma functionality.

