Enterprise Content Management System (ECMS) | Document Management System (DMS)
Digitization is impacting every industry. Businesses have more need than ever to unleash the power of content that is generated across the organization(s). Content/documents are not anymore static pieces of paper in today’s business. True Life Cycle Management of the content is crucial for key success of any business. Publishing the right (approved) content that drives the business in right direction needs true collaboration across the authors, approvers and consumers of the content working in different time zones and geographical places along with the tight security.
In today’s world all the content (digital assets; Standard Operating Procedures; White Papers; Knowledge Base articles; Spread Sheets; etc.) need to be created, approved, published and maintained right within the people and make these assets available across all the devices to boost the efficiency.
Atachi ECMS is a true Enterprise Content Management System that completely leverages the CMIS (Content Management Information System) features along with the Cloud and In-Memory technologies.
At Atachi we understand that your content shouldn’t get locked in any particular repository and generate digital silos by replacing paper silos. Atachi ECMS completely integrates with other Enterprise content systems like box.com and Office 365. With this integration built-in as a customer you have complete flexibility to transport and/collaborate the data across these systems within the enterprise(s).
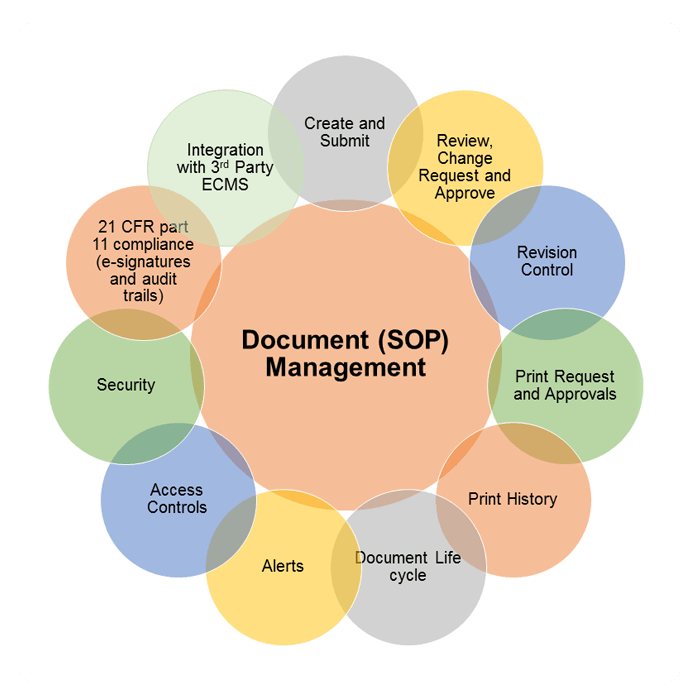
DMS for Pharma Industry | SOP Management for Pharma | DMS for Regulated/Manufacturing Industry
Atachi DMS powered by SAP/HANA cloud platform provides electronic document management in an efficient and cost effective manner. Cloud based in memory platform makes it accessible on different types of devices ranging from computers to smart phones. Atachi DMS is a 21 CFR part 11 compliant product that makes it more secure than any other document management system. Atachi DMS is suitable for small and medium size business that provide security to their documents and works in a team. Atachi DMS is designed to promote cGMP (Current good manufacturing practice). As part of CGMP, ATACHI DMS can create, update, review, approve Standard Operating Procedures (SOP) and reduce paper.
Atachi DMS for Pharma also manages version control of the SOP document. Version control of DMS keep track of every modification to the document. It helps the management to compare the earlier versions, who changes which document and when, with full roll back to a previous version if needed. The control of both the content and information about the documents removes unauthorized access.
Atachi DMS for Pharma has the ability to manage the document processing, workflow, history and retrieval processes. It empowers business organizations to get a complete control over the documents and ensure quick retrieval of the content.
The Unique ID created by Atachi DMS for Pharma will give an easy access for Document history and reference document tracking.
Electronic documents are signed prior to approval and publishing to the document tree. Electronic signatures including name, date, time and comments are captured automatically to each document. This makes monitoring changes to the data easier
Atachi DMS captures all document processes, from the original author to any changes made to the document. All revisions are kept and available for review or auditing purposes.
Atachi DMS is a cloud based as it can connect all employees involved in document and quality control from virtually anywhere. It provides easy access, search and retrieval of electronic documents by all authorized users.
Users can be controlled for electronic document printing by sending requests for printing approval. The Unique ID created by Atachi DMS for Pharma keeps tracking of the printing history.
Electronic document expiry dates are maintained and the automatic notifications by SMS/email are sent to applicable personnel to take intelligent action. Atachi DMS provides the ability to escalate the action to ensure appropriate follow-up.