Atachi QMS has an in built intelligence to capture documents processes, procedures, and responsibilities for achieving quality policies with strict adherence to GMP (Good Manufacturing Practices) and objectives of an organization.
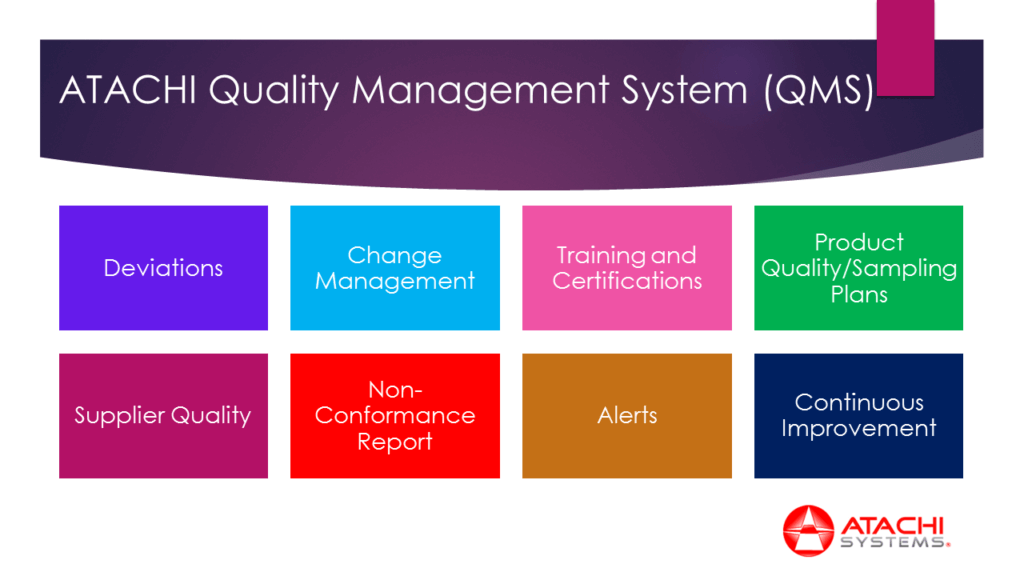
- Sampling Plans
Atachi Quality Management System (QMS) regulates quality, deviations from the stipulated quality parameters and other quality related issues.
- Deviations
Atachi QMS has the ability to integrate and monitor the process deviations, environmental parameters, operational deviations and equipment deviations to take immediate intelligent action(s).
- Continuous Improvement
Atachi QMS serves many purposes to organizations including improving processes, reducing waste, lowering cost, facilitating and identifying training opportunities. Atachi QMS produces best practices for controlling product and process outcomes were established and documented.
- Operator Training and Certifications
Your organization can introduce enforcement of certification for process steps through Atachi QMS by separating roles with different certifications. Atachi QMS has the ability to provide the wide range of video bases and document based training for their customers.
- Change Management
Atachi QMS has the ability to manage the change management by providing the controls like change, review, approval, execute…etc with in the groups and the roles. Atachi QMS enables your organization to achieve the goals and objectives set out in its policy and strategy. It provides consistency and satisfaction in terms of methods, materials, equipment, etc, and interacts with all activities of your organization.
- NCR and Alerts
Creation of Non-conformance report during the deviation with in the set limits, automatic notifications by SMS/email to applicable personnel for immediate action is part and parcel of Atachi QMS.
- Regulations/ Compliance
Atachi QMS implementation affects every aspect of an organization’s performance. It helps in meeting the customer requirements which instill confidence in the organization which turns into leading to more customers, more sales and more repeat business. It ensure compliance with the regulations creating room for expansion, growth and profit.
- End-To-End Quality
Atachi QMS gives you end to end quality management solutions. It addresses the compliance with the regulations depending on the customer requirements. Atachi QMS has the ability to integrate with PLC, ERP and other systems to capture the data in real time. Atachi QMS bring you the day to day business intelligence for the complete manufacturing process in real time.
QMS for Pharmaceutical Manufacturing | QMS for API Manufacturers
Atachi QMS (Quality Management System) for Pharmaceutical Manufacturing has built on the International Conference on Harmonization guidelines ICH Q10, cGMP (common Good Manufacturing Practices) and FDA 21 CFR Part 11 guidelines. At Atachi, we have built the QMS has strict adherence to the the ICH Q7 guidelines for API (Active Pharmaceutical Ingredient) Manufacturers. Our QMS is implemented and/have most of in-built controls for the Quality Risk Management as per the guidelines of ICH Q9 is properly managed at every step of the manufacturing processes along with facilities, equipment, environment and personnel.
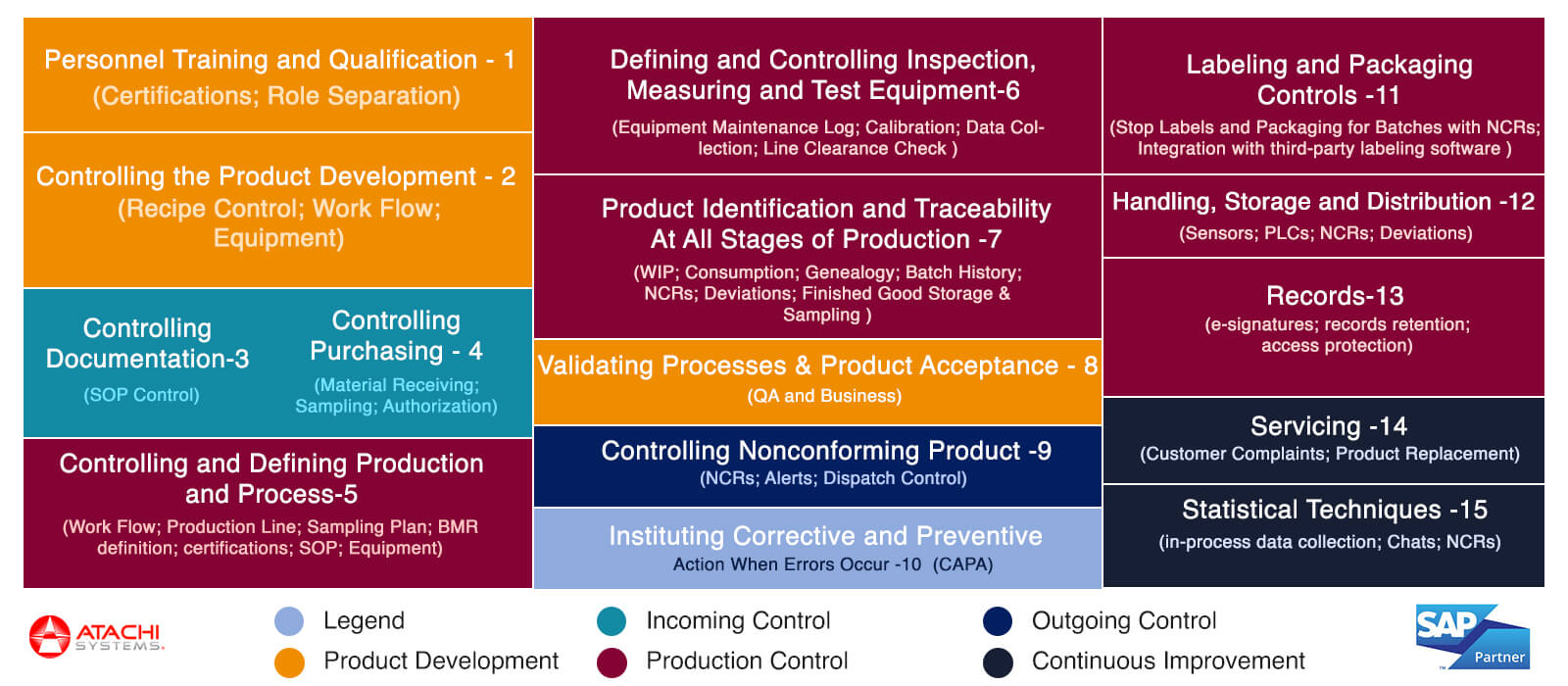
Please see the below screenshot for the complete coverage of Atachi QMS for Pharmaceutical Quality Management system functionalities ICH Q10.
NGIMES Coverage for PQS (QMS)